Chapter 2 - Transmission Based Precautions (TBPs)
Transmission-based precautions (TBPs) definitions literature review update
What is changing in this update?
New research questions
New research questions have been considered as part of this update. These look at:
- the historical evidence behind the current transmission descriptors (contact, droplet, airborne) and whether these are still fit for purpose
- how infectious agents are released into the air of the health and care environment from the respiratory tract with consideration of particle size, distance and clearance/fallout time
- the evidence for aerosol generating procedures
New search terms
New search terms have been added. The evidence search goes back to include all scientific papers that have been published since the year 2000.
See the NIPCM literature review development process for more information.
See the full list of research questions considered and the search terms within the revised literature review. Part A of the Considered Judgement Form has been published alongside the revised literature review to provide a summary of the evidence. The review does not contain any recommendations for practice as these are still being developed by the ARHAI Scotland National Policies Guidance and Evidence (NPGE) Working Group.
Due to the scope and complexity of this work, additional external specialist engagement required, and stakeholder involvement to date, ARHAI Scotland have revised the timelines for this review. It is anticipated that the recommendations for practice will be developed during the summer months of 2025, with a revised implementation phase commencing in spring 2026
Evidence for mask wearing
Stakeholders requested that this review also considers where and when healthcare workers should wear masks and the type of mask they should wear (surgical mask or respirator). Evidence for mask effectiveness is being considered in separate literature review updates .
Why are transmission descriptors (contact, droplet, airborne) being reviewed?
The pandemic highlighted that the way in which respiratory transmission is currently described (droplet and airborne transmission) may not reflect what is happening in real life. We need to look at whether there is a better way to describe transmission, and whether this would lead to any improvements in infection prevention and control (IPC) practice.
Understanding how infectious agents are released into the air and the risks associated with particle size and distance from source will help inform this. Reviewing the evidence to understand if there is increased risk associated with certain medical procedures will also inform IPC practice.
The World Health Organization (WHO) and Centers for Disease Control (CDC) have also reviewed transmission descriptors indicating a global shift in the way transmission routes are described. ARHAI Scotland were invited to meet with the WHO global IPC unit to discuss the topic and our literature review findings were well received.
Why were transmission descriptors not reviewed during the pandemic?
Work began on reviewing transmission descriptors in 2022 in the third year of the pandemic. The literature review has assessed over 26,000 scientific articles. 61% of all studies included to answer the main research question were published between 2019 and 2022 which reflects the growing interest in this area as the pandemic continued.
What is likely to change in the NIPCM?
The ARHAI Scotland National Policies Guidance and Evidence (NPGE) Working Group are currently developing recommendations for practice. It is likely that ‘droplet transmission’ and ‘airborne transmission’ will be replaced with new definitions to describe respiratory transmission. This will mean changes throughout the NIPCM to update the terminology including the addition of resources to support any guidance changes.
What might this mean for healthcare workers in practice?
It is too early to understand what might change in practice but it is likely that there will be a need for healthcare workers to consider more factors when risk assessing what PPE to wear.
The goal of the NIPCM is to provide healthcare workers in Scotland with guidance that is evidence based, up-to-date, effective, practical, and as a result, safe. There should be a clear benefit associated with any guidance change and this benefit should outweigh any potential harms. Guidance will only change if these conditions are met.
Supporting resources and education needs will be considered alongside any potential changes to the NIPCM to enable application to practice.
About transmission based precautions (TBPs)
SICPs may be insufficient to prevent cross-transmission of specific infectious agents. Therefore, additional precautions known as transmission based precautions (TBPs) are required to be used by staff when caring for patients with a known or suspected infection or colonisation.
Transmission routes
TBPs are categorised by the route of transmission of infectious agents. Some infectious agents can be transmitted by more than one route.
Contact precautions
Used to prevent and control infections that spread via direct contact with the patient or indirectly from the patient’s immediate care environment (including care equipment). This is the most common route of cross-infection transmission.
Droplet precautions
Used to prevent and control infections spread over short distances (at least 3 feet or 1 metre) via droplets (greater than 5μm) from the respiratory tract of one individual directly onto a mucosal surface or conjunctivae of another individual. Droplets penetrate the respiratory system to above the alveolar level.
Airborne precautions
Used to prevent and control infections spread without necessarily having close patient contact via aerosols (less than or equal to 5μm) from the respiratory tract of one individual directly onto a mucosal surface or conjunctivae of another individual. Aerosols penetrate the respiratory system to the alveolar level.
Application of TBPs
Clinical judgement and decisions should be made by staff on the necessary precautions. This must be based on the:
- suspected or known infectious agent
- transmission route of the infectious agent
- care setting and procedures undertaken
- severity of the illness caused
Appendix 11 provides details of the type of precautions, optimal patient placement, isolation requirements and any respiratory precautions required.
Application of TBPs may differ depending on the setting and the known or suspected infectious agent.
Further information on Transmission Based Precautions can be found in the definitions of Transmission Based Precautions literature reviews.
Last updated: 28 August 2023
2.1 Patient Placement/Assessment for Infection Risk
The potential for transmission of infection must be assessed at the patient’s entry to the care area. If hospitalised or in a care home setting this should be continuously reviewed throughout the stay/period of care. The assessment should influence placement decisions in accordance with clinical/care need(s).
Patients who may present a cross-infection risk in any setting includes but is not limited to those:
- with symptoms such as loose stools or diarrhoea, vomiting, fever or respiratory symptoms.
- with a known (laboratory confirmed) or suspected infectious pathogen for which appropriate duration of precautions as outlined in A-Z of pathogens are not yet complete
- known or suspected to have been previously positive with a Multi-drug Resistant Organism (MDRO), for example MRSA, CPE
- who have been hospitalised (inpatient) outside Scotland in the last 12 months (including those who received dialysis)
Further information regarding general respiratory screening questions can be found within the resources section of the NIPCM.
Isolation facilities should be prioritised depending on the known/suspected infectious agent (refer to Aide Memoire - Appendix 11). All patient placement decisions and assessment of infection risk (including isolation requirements) must be clearly documented in the patient notes.
When single-bed rooms are limited, patients who have conditions that facilitate the transmission of infection to other patients (e.g., draining wounds, stool incontinence, uncontained secretions) and those who are at increased risk of acquisition and adverse outcomes resulting from HAI (e.g., immunosuppression, open wounds, invasive devices, anticipated prolonged length of stay, total dependence on HCWs for activities of daily living) should be prioritised for placement in a single-bed room. Single-bed room prioritisation should be reviewed daily and the clinical judgement and expertise of the staff involved in a patient's management and the Infection Prevention and Control Team (IPCT) or Health Protection Team (HPT) should be sought particularly for the application of TBPs e.g. isolation prioritisation when single rooms are in short supply.
Hospital settings
- Patients who present a cross-infection risk should be isolated in a single room or for patients with a known or suspected pathogen spread by the airborne route, in a specialised negative pressure isolation facility where available.
- Isolation of infectious patients can be in specialised isolation facilities, single room isolation, cohorting of infectious patients where appropriate, ensuring that they are separated by at least 2 metres with the door closed.
- Signage should be used on doors/areas to communicate isolation requirements and prevent entry of unnecessary visitors and non-essential staff.
- Infectious patients should only be transferred to other departments if medically necessary. If the patient has an infectious agent transmitted by the airborne/droplet route, then if possible/tolerated the patient should wear a surgical face mask during transfer.
- Receiving department/hospital and transporting staff must be aware of the necessary precautions.
Cohorting in hospital settings
Cohorting of patients
Cohorting of patients should only be considered when single rooms are in short supply and should be undertaken in conjunction with the local IPCT.
Patients who should not be placed in multi bed cohorts:
- patients with different infectious pathogens/strains and patients with unknown infectious pathogens (laboratory confirmation still awaited)
- patients considered more vulnerable to infection
- patients with a known or suspected infectious pathogen spread by the droplet/airborne route who will undergo an AGP
- patients who are unlikely to comply with TBPs
Staff cohorting
Consider assigning a dedicated team of care staff to care for patients in isolation/cohort rooms/areas as an additional infection control measure during outbreaks/incidents. This can only be implemented through planning of staff rotas if there are sufficient levels of staff available to ensure consistency in staff allocation (so as not to have a negative impact on non-affected patients’ care).
Before discontinuing isolation in hospital settings
Individual patient risk factors should be considered, for example there may be prolonged shedding of certain microorganisms in immunocompromised patients). Clinical and molecular tests to show the absence of microorganisms may be considered in the decision to discontinue isolation and can reduce isolation times. The clinical judgement and expertise of the staff involved in a patient’s management and the Infection Prevention and Control Team (IPCT) or Health Protection Team (HPT) should be sought on decisions regarding isolation discontinuation.
Primary care/out-patient settings
- Patients attending these settings with suspected/known infection/colonisation should be prioritised for assessment/treatment, for example scheduled appointments at the start or end of the clinic session. Infectious patients should be separated from other patients whilst awaiting assessment and during care management wherever possible.
- If transfer from a primary care facility to hospital is required, the ambulance service should be informed of the infectious status of the patient.
Resources
Further information can be found in the patient placement literature review.
2.2 Safe Management of Patient Care Equipment in an Isolation Room/Cohort Area
- Use single-use items if possible.
- Reusable non-invasive care equipment should be dedicated to the isolation room/cohort area and decontaminated prior to use on another patient Section 1.5. Safe Management of Care Equipment
- An increased frequency of decontamination should be considered for reusable non-invasive care equipment when used in isolation/cohort areas.
If an item cannot withstand chlorine releasing agents staff are advised to consult the manufacturer’s instructions for a suitable alternative to use following or combined with detergent cleaning.
Resources
For how to decontaminate non-invasive reusable equipment see Appendix 7.
Note: Scottish Ambulance Service (SAS) and Scottish National Blood Transfusion Service adopt practices that differ from those stated in the National Infection Prevention and Control Manual.
2.3 Safe Management of the Care Environment
Routine environmental decontamination
Hospital/care home setting
Patient isolation/cohort rooms/area must be decontaminated at least daily, this may be increased on the advice of IPCTs/HPTs. These areas must be decontaminated using either:
- a combined detergent/disinfectant solution at a dilution of 1,000 parts per million available chlorine (ppm available chlorine (av.cl.)) or
- a general purpose neutral detergent in a solution of warm water followed by disinfection solution of 1,000ppm av.cl.
Manufacturers’ guidance and recommended product "contact time" must be followed for all cleaning/disinfection solutions .
Increased frequency of decontamination/cleaning schedules should be incorporated into the environmental decontamination schedules for areas where there may be higher environmental contamination rates, for example
- toilets/commodes particularly if patients have diarrhoea
- “frequently touched” surfaces such as door/toilet handles and locker tops, over bed tables and bed rails
Patient rooms must be terminally cleaned following resolution of symptoms, discharge or transfer. This includes removal and laundering of all curtains and bed screens.
Vacated rooms should also be decontaminated following an AGP.
Primary care/out-patient settings
The extent of decontamination between patients will depend on the duration of the consultation/assessment, the patients presenting symptoms and any visible environmental contamination.
Equipment used for environmental decontamination must be either single-use or dedicated to the affected area then decontaminated or disposed of following use for example cloths, mop heads.
Terminal decontamination
Following patient transfer, discharge, or once the patient is no longer considered infectious.
Remove from the vacated isolation room/cohort area, all:
- healthcare waste and any other disposable items (bagged before removal from the room)
- bedding/bed screens/curtains and manage as infectious linen (bagged before removal from the room)
- reusable non-invasive care equipment (decontaminated in the room prior to removal) Appendix 7.
The room should be decontaminated using either:
- a combined detergent disinfectant solution at a dilution (1,000ppm av.cl.) or
- a general purpose neutral detergent clean in a solution of warm water followed by disinfection solution of 1,000ppm av.cl..
The room must be cleaned from the highest to lowest point and from the least to most contaminated point.
Manufacturers’ guidance and recommended product "contact time" must be followed for all cleaning/disinfection solutions .
Unless instructed otherwise by the IPCT there is no requirement for a terminal clean of an outpatient area or theatre recovery.
Note: Scottish Ambulance Service (SAS) and Scottish National Blood Transfusion Service adopt practices that differ from those stated in the National Infection Prevention and Control Manual.
When an organisation adopts practices that differ from those recommended/stated in the NIPCM with regards to cleaning agents, the individual organisation is fully responsible for ensuring safe systems of work, including the completion of local risk assessment(s) approved and documented through local governance procedures.
2.4 Personal Protective Equipment (PPE): Respiratory Protective Equipment (RPE)
2.4.1 Surgical masks
A type IIR fluid resistant surgical mask should be worn when caring for a patient with a suspected/confirmed infectious agent spread by the droplet route.
Surgical masks worn by patients with suspected/confirmed infectious agents spread by the droplet or airborne routes, as a form of source control, should meet type II or IIR standards.
2.4.2 Eye/face protection
Eye and face protection should be worn in combination with:
- a fluid resistant type IIR surgical mask when caring for symptomatic patients infected with droplet transmitted infectious agents
- a fluid resistant FFP3 respirator when caring for symptomatic patients infected with an airborne transmitted infectious agent
Eye and face protection should be worn:
- when there is an anticipated risk of splashing and/or spraying of blood or bodily fluids, and
- when caring for patients with novel infectious agents including pandemic influenza
2.4.3 Aprons/Gowns
An apron should be worn when caring for patients known or suspected to be colonised/infected with antibiotic resistant bacteria including contact with the patient’s environment.
Plastic aprons should be used in health and social care settings for protection against contamination with blood and/or body fluids.
A fluid repellent gown should be used if excessive splashing or spraying is anticipated.
A full body fluid repellent gown should be worn when conducting AGPs on patients known or suspected to be infected with a respiratory infectious agent.
Resources
Further information can be found in the Aprons/Gowns literature review.
2.4.4 Gloves
Gloves must:
- be worn when exposure to blood, body fluids, (including but not limited to secretions and/or excretions), non-intact skin, lesions and/or vesicles, mucous membranes, hazardous drugs and chemicals, e.g. cleaning agents is anticipated/likely
- Gloves are a single-use item and should be donned immediately prior to exposure risk and should be changed immediately after each use or upon completion of a task
- never be worn inappropriately in situations such as to go between patients, move around a care area, work at IT workstations
- be changed if a perforation or puncture is suspected or identified
- be appropriate for use, fit for purpose and well-fitting
- not be worn as a substitute to hand hygiene
Double gloving is only recommended during some Exposure Prone Procedures (EPPs), for example orthopaedic and gynaecological operations, or when attending major trauma incidents and when caring for a patient with a suspected or known High Consequence Infectious disease. Double gloving is not necessary at any other time.
Resources
For appropriate glove use and selection see Appendix 5.
Further information can be found in the Gloves literature review.
2.4.5 RPE
PPE must still be used in accordance with SICPs when using Respiratory Protective Equipment. See Chapter 1.4 for PPE use for SICPs.
Where it is not reasonably practicable to prevent exposure to a substance hazardous to health (as may be the case where healthcare workers are caring for patients with suspected or known airborne micro-organisms) the hazard must be adequately controlled by applying protection measures appropriate to the activity and consistent with the assessment of risk. If the hazard is unknown the clinical judgement and expertise of IPC/HP staff is crucial and the precautionary principle should apply.
Respiratory Protective Equipment (RPE), for instance FFP3 and facial protection, must be considered when:
- a patient is admitted with a known/suspected infectious agent/disease spread wholly by the airborne route
- carrying out aerosol generating procedures (AGPs) on patients with a known/suspected infectious agent spread wholly or partly by the airborne or droplet route
See Appendix 16 for the extant list of Aerosol Generating Procedures which require the application of airborne precautions and details of associated Post AGP Fallow times.
Filter Face Piece 3 (FFP3) Respirators
Where staff have concerns, they may choose to wear an FFP3 respirator rather than a fluid-resistant surgical mask (FRSM) when providing patient care, provided they are fit tested. This is a personal PPE risk assessment.
All tight fitting RPE (for instance FFP3) respirators must be:
- fit tested (by a competent fit test operator) on all healthcare staff who may be required to wear a respirator to ensure an adequate seal/fit according to the manufacturers’ guidance
- fit checked (according to the manufacturers’ guidance) every time a respirator is donned to ensure an adequate seal has been achieved. The poster below gives further information on compatibility of facial hair and FFP3 respirators and can be used when fit testing and fit checking
- single use (disposable) and fluid-resistant. Valved respirators may be shrouded or unshrouded. Respirators with unshrouded valves are not considered to be fluid-resistant and therefore should be worn with a full face shield if blood or body fluid splashing is anticipated
- non valved if a sterile procedure is being performed at the same time as an AGP requiring a respirator to be worn. An MHRA safety alert can be viewed.
- compatible with other facial protection used, for instance protective eyewear, so that this does not interfere with the seal of the respiratory protection. Prescription eyeglasses and contact lenses should not be considered a form of eye/face protection. If wearing a valved, non-shrouded FFP3 respirator a full face shield/visor must be worn
- always put on before entry into the patient room/area and prior to performing an aerosol generating procedure (AGP) and removed in an anteroom/lobby or in a safe area, for example outside the isolation/cohort room/area. All other PPE should be removed in the patient care area
- changed after each use. Other indications that a change in respirator is required include: if breathing becomes difficult, if the respirator becomes wet or moist, damaged or obviously contaminated with body fluids such as respiratory secretions.
Resources
Poster on compatibility of facial hair and FFP3 respirators can be used when fit testing and fit checking.
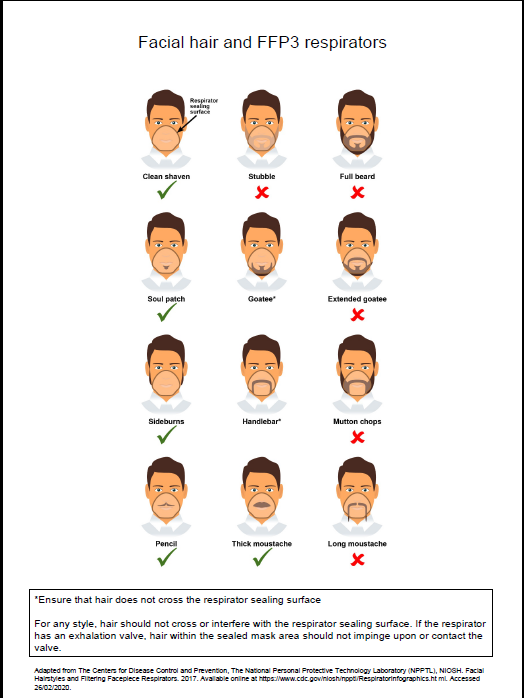
Further information regarding fitting and fit checking of respirators can be found on the Health and Safety Executive website.
National Priority Risk Categorisation for face fit testing with FFP3
The following risk categorisation is the minimum requirement for staff groups that require FFP3 fit testing. NHS boards can add to this for example where high-risk units are present. This categorisation is inclusive of out of hours services.
Level 1 – Preparedness for business as usual
Staff in clinical areas most likely to provide care to patients who present at healthcare facilities with an infectious pathogen spread by the airborne route; and/or undertake aerosol generating procedures. These are A&E, ICU, paediatrics, respiratory, infectious diseases, anaesthesia, theatres, Chest physiotherapists, Special Operations Response Team (Ambulance), A&E Ambulance Staff, Bronchoscopy Staff, Resuscitation teams, mortuary staff.
Level 2 – Preparedness in the event of emerging threat
Staff in clinical setting likely to provide care to patients admitted to hospital in the event of an emerging threat, for example Medical receiving, Surgical, Midwifery and Speciality wards, all other ambulance transport staff.
In the event of an ‘Epidemic/Pandemic’ Local Board Assessment as per their preparedness plans will apply.
The decision to wear an FFP3 respirator/hood should be based on clinical risk assessment, for example task being undertaken, the presenting symptoms, the infectious state of the patient, risk of acquisition and the availability of treatment.
Resources
For a list of organisms spread wholly or partly by the airborne (aerosol) or droplet routes see Appendix 11.
Further information can be found in the aerosol generating procedures literature review.
Powered respirator hoods
Powered respirator hoods are an alternative to FFP3 respirators for example when fit testing cannot be achieved.
Powered hoods must be:
- single use (disposable) and fluid resistant
- the filter must be enclosed with the exterior and the belt able to withstand disinfection with 10,000ppm av.cl.
FFP3 respirator or powered respirator hood
- may be considered for use by visitors if there has been no previous exposure to the infected person or infectious agent; but
- must never be worn by an infectious patient(s) due to the nature of the respirator filtration of incoming air not expelled air.
Work is currently underway by the UK Re-useable Decontamination Group examining the suitability of respirators for decontamination. This literature review will be updated to incorporate recommendations from this group when available. In the interim, ARHAI Scotland are unable to provide assurances on the efficacy of respirator decontamination methods and the use of re-useable respirators is not recommended.
Further information can be found in the Respiratory Protective Equipment (RPE) literature review and the Personal Protective Equipment (PPE) for Infectious Diseases of High Consequence (IDHC) literature review.
2.5 Infection Prevention and Control during care of the deceased
The principles of SICPs and TBPs continue to apply whilst deceased individuals remain in the care environment. This is due to the ongoing risk of infectious transmission via contact although the risk is usually lower than for living patients.
It is important that information on the infection status of the deceased is sought and communicated at each stage of handling. Appropriate risk assessment must be carried out before performing activities that may increase the risk of transmission of infectious agents from deceased individuals (see literature review for further information on these activities).
Washing and/or dressing should not be carried out when the deceased is known or suspected to have been infected by any of the following key infectious agents: Hazard Group 4 organisms, anthrax, and rabies. For other HCIDs a local risk assessment should be undertaken to inform any decision making on washing and/or dressing of the deceased.
Viewing of the deceased should be avoided when the deceased is known or suspected to have been infected by Hazard Group 4 organisms, specifically those causing VHFs (including Ebola, Lassa etc.) and anthrax. For other HCIDs a local risk assessment should be undertaken to inform any decision making on viewing of the deceased.
See Appendix 12 Application of infection control precautions in the deceased.
Staff should advise relatives of the appropriate precautions when viewing and/or having physical contact with the deceased including when this should be avoided.
Deceased individuals known or suspected to have a Hazard Group 4 infectious agent should be placed in a sealed double plastic body bag with absorbent material placed between each bag. The surface of the outer bag should then be disinfected with 1000 ppm av.cl before being placed in a robust sealed coffin.
Post-mortem examination should not be performed on a deceased individual known or suspected to have Hazard Group 4 infectious agents. See Appendix 12 Application of infection control precautions in the deceased. Blood sampling can be undertaken in the mortuary by a competent person to confirm or exclude this diagnosis. Refer to Section 2.4 for suitable PPE.
Post-mortem examination of deceased individuals known or suspected to have been infected by transmissible spongiform encephalopathies (TSE) causing agents should be carried out in such a way as to minimise contamination of the working environment. See Literature review for further information.
Content
- Transmission-based precautions (TBPs) definitions literature review update
- About transmission based precautions (TBPs)
- 2.1 Patient Placement/Assessment for Infection Risk
- 2.2 Safe Management of Patient Care Equipment in an Isolation Room/Cohort Area
- 2.3 Safe Management of the Care Environment
- 2.4 Personal Protective Equipment (PPE) and Respiratory Protective Equipment (RPE)
- 2.5 Infection Prevention and Control during care of the deceased

